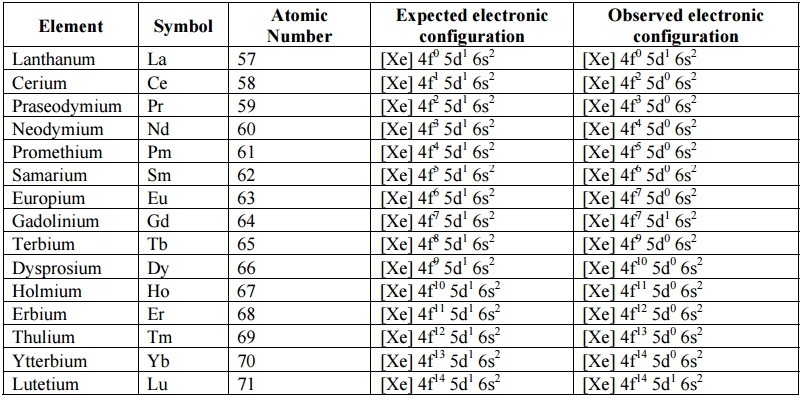
In the lanthanoids, 4f and 5d subshells are very close in energy. The outermost 6s-orbital remains filled with two electrons (6s2) . The electrons can easily jump from 4f to 5d or vice-versa. Further, irregularities in electronic configurations are also related to the stabilities of f0 f7 and f14 occupancy of f-orbitals. Hence, their electronic configurations are not known with certainty.
Electronic configuration of lanthanum and 4f-series of f-block elements:
i. The 4f-series includes elements from cerium (Ce) to lutetium (Lu). The electronic configuration of these elements can be expressed in terms of its nearest inert gas Xe (Z = 54).
ii. Electronic configuration of Xe (Z = 54) = 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 . Therefore, general electronic configuration of 4f-series is [Xe] 4f 1-14 5d 0-1 6s2
iii. Lanthanum has electronic configuration [Xe] 4f0 5s1 6s2 . It does not have any 4f electrons.
For more Q & A of any subject of Maharashtra HSC Board CLICK HERE
One thought on “Briefly explain why electronic configurations of lanthanoids are not known with certainty.”